
Water Quality and Spray Tank Implications
When was the last time you tested the water source you use to fill up your sprayer?
Something very common across agriculture is the lack of understanding of how water in a spray tank can impact the overall efficacy of spray material. Spray water pH and the number of dissolved minerals (hardness) have a direct impact on many of the crop protection products we use. Most of us fail to realize that local water source accounts for more than 98% of the total spray tank mixture and once you put pesticides into bad water, the damage is done and can’t be reversed. I bet I can ask any of you mixing a tank this week what you paid per gallon for the crop protection you’re using and I’ll get a quick answer. Now let me also ask how much total investment you have in each spray tank load. That’s an investment we need to protect so what are the areas we should be focused on?
Spray water pH and crop protection pH sensitivity
Certain spray materials break down if spray water is too basic (high pH) or acidic (low pH). Generally, the most optimum range for spray water pH is 5-6.5 with very few product outliers. Throughout most of the San Joaquin and Sacramento Valley water pH ranges widely but on average above 7.5 pH and in some areas close to 9pH. Well, what happens to your spray materials at that pH? Many of the fungicides and insecticides we use degrade rapidly (alkaline hydrolysis) at that pH especially if that tank mix sits for any length of time. Below is a study about the effects of pH on the half-life of Imidan Insecticide. With just 3 points of pH change, the half-life goes from 178 hours to 33 minutes. How many of your employees take 30-minute lunches with a full or partially full spray tank? How long does it take for your sprayer to drive from the loading pad to the field and actually start spraying? If you haven’t buffered your spray water down the damage is being done. Products such as Malathion, Sevin, Apollo®, Captan, Dimethoate, Paraquat, and many others are susceptible to the same loss mechanism. It is highly recommended to pull a water sample from the source you use to fill spray tanks to accurately buffer to a desired pH.
Effects of pH on Imidan Half-Life
| pH | Half-Life |
|---|---|
| 5.0 | 178 Hours |
| 5.5 | 92 Hours |
| 6.0 | 36 Hours |
| 6.5 | 14 Hours |
| 7.0 | 10 Hours |
| 7.5 | 2 Hours |
| 8.2 | 33 Minutes |
How do we mitigate high spray water pH?
Common buffering agents use a citric acid base to lower the total spray tank pH at low rates. We commonly recommend TRI-FOL® for this at 8-16oz/100 gallons and have easy-to-use test kits that will help you know exactly how much TRI-FOL you need to buffer down to a desired pH. Another way to tackle this problem is to use a combination product that contains an excellent non-ionic surfactant and will buffer down the spray tank mixture. We recommend DENALI-EA® in situations that require an excellent surfactant along with a buffering or acidifying effect to the final spray mixture.


Spray solution and crop protection mineral antagonism
Water hardness or mineral antagonism can directly interfere with a large range of the pesticides we use. So what is mineral antagonism? Mineral antagonism is dissolved minerals in our water sources comprising mainly of Calcium (Ca++), Magnesium (Mg++), Iron (Fe++), and Sodium (Na+). What is something you see in common with these 4 minerals? Hopefully, you answered that they all share a positive charge. What’s critically important about that is a majority of our pesticides carry a negative charge. Because positives and negatives attract, that mineral antagonism begins the moment we start adding active ingredients to the spray tank ultimately bonding together and rendering a percentage of our active ingredient useless. So how do we mitigate for mineral antagonism?
The Addition of Ammonium Sulfate & Why
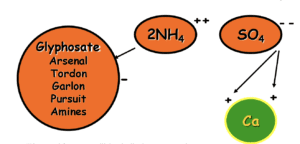
The Sulfate ion “blocks” the Mineral cation.
The Ammonium ions attach and “escort” the glyphosate into the plant.
The addition of ammonium sulfate-based material or AMADs will solve this problem but must be added to the spray tank before adding pesticides. Ammonium Sulfate and AMADs work because the sulfate molecule (SO4- -) has 2 negative charges which readily associate with the positively charged mineral in our spray tank. The ammonium molecule (2NH4 + +) weakly associates with our negatively charged active ingredients and helps to more efficiently escort the active into the plant tissue. Even in areas without hard water ammonium sulfate is used to improve the uptake of pesticides. A very common recommendation from Wilbur-Ellis when dealing with mineral antagonism is to use Bronc Max (AMS solution) at 2-4 quarts per 100 gallons if we only need to mitigate for water hardness. Another option is to use Cayuse Plus which contains a high-quality NIS and AMS solution.
Regardless of which products you are using to condition your spray water, the important thing is YOU’RE TREATING YOUR SPRAY WATER! The last thing any of us want to do is flush our investment down the drain and not get the maximum performance out of our pesticide products because the most expensive application you will make all year is the one you have to go back and do again.
If you need help on how to sample your spray water, where to send the sample for analysis, or how to interpret the results please reach out to your local Wilbur-Ellis PCA.

